How to Reading Wavelength Graphs on a Spectroscope
How to Measure a Spectrum
Breaking Light into a Spectrum: Dispersion and Diffraction
Simply well-nigh every astronomy textbook you will ever pick up volition contain a phrase to the effect that the process of breaking light upwards into a spectrum is "similar passing white calorie-free through a prism." This process, chosen dispersion, arises considering different colors (or wavelengths) of light bend by different amounts as they pass from, say, a low density medium (like air) into a higher density medium (like the glass in a prism). Hence, a narrow beam of "white" calorie-free will get spread out into a rainbow. Voila, a spectrum!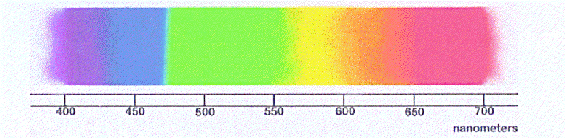
The colors of the familiar "rainbow" of visible light correspond to differing wavelengths of the light, here shown on a nanometer scale. The wavelengths get successively larger as ane moves from left to right.
But such a spectrum, although very pretty, is of very petty use to astronomers. This kind of spectrum does non convey the detailed physical information that we require to do science. And every bit a practical matter, some kinds of light (such equally ultraviolet low-cal for instance) do not pass through a glass prism just rather are absorbed! It is tough to measure a spectrum when the light gets absorbed!
In practice, nigh spectrographs in astronomy, including those that operate in the optical part of the spectrum, apply a totally dissimilar method for creating a spectrum out of the incoming light from the telescope--the procedure of diffraction. This process depends on the wave-similar properties of the calorie-free, and uses a component called a diffraction grating to actually split the calorie-free into its component wavelengths. A diffraction grating consists of a substrate (often fabricated of glass, but stainless steel, plastic, or other materials are sometimes used) onto which are etched very narrowly-spaced lines. How narrowly-spaced? Well, a typical diffraction grating used in optical astronomy may have anywhere from several hundred to over one k lines etched per millimeter! A famous physicist from Johns Hopkins University, Henry A. Rowland, was the commencement person to make high quality diffraction gratings for employ in scinece.
How does such a grating intermission a axle of calorie-free into its component wavelengths? [TBD. Hope to add together this soon! But most Intro Physics text books give a description of this procedure. So until I get around to information technology, utilize the Library!]
Spectroscopes and Spectrographs
A diffraction grating by itself is really no better than a prism for creating an astronomical spectrum. The grating must be built into a device called a spectroscope or spectrograph for this to be washed. These are finer the same matter except that a spectroscope is simply used for visual inspection (that is, your heart is the detector), while a spectrograph includes some means (photographic pic or an electronic detector) for recording the spectrum for analysis. In professional astronomy these days at that place is very little need for a spectroscope (just as there is very niggling other observational work really done with the naked centre, with the possible exception of staring at a computer monitor all day!).Ok, so what is a spectrograph? In its simplest form, it is a light-tight box with a modest (often narrow rectangular or adjustable) opening to let light in, a grating to break the light into its components, and a "detector" of some kind placed at the proper angle and distance from the grating to record the spectrum of the wavelength range of interest. Telescopes are used to get together the faint low-cal from distant objects, and the spectrographs are placed at the focus of the telescope to clarify the calorie-free.
Detecting and Recording Spectra
A detector is simply a device that senses and measures the incoming lite. In a spectrograph, the detector has to perform this task beyond a range of wavelengths, measuring the amount of low-cal every bit information technology changes from wavelength to wavelength. In an optical spectroscope, the detector is your eye, which senses the different colors and the presence of dark absorption lines or brilliant emission lines in the spectrum of the source existence viewed. In a spectrograph, some other device is used to sense the calorie-free.For many years the primary detector used in spectrographs was the photographic plate (basically moving-picture show, although special astronomical emulsions placed on glass plates were used for greater sensitivity and stability). Oft spectra recorded this way were so traced with a device chosen a (are yous ready for this?) microdensitometer. This device would shine a steady, narrow beam of calorie-free through the photographic plate to a lite sensitive photomultiplier tube. As the plate was stepped forth the length of the spectrum, the photomultiplier tube would measure and record the amount of light at each wavelength. The resulting tracing would essentially be a graph of the intensity of calorie-free as a part of the position on the photographic plate (or as a function of wavelength in the case of a spectrum). This graphical representation of a spectrum is what astronomers find near useful in doing their work.
Over the last xx years or so even photographic recording of spectra has nearly get a thing of the past. Electronic recording of spectra is the almost sensitive, quantitative, way of detecting the low-cal, and information technology gets the spectrum directly into a digital form that can be handled on a computer (where the existent work gets washed). The detector used about often in astronomy these days is called a accuse coupled device, or CCD. This device is basically an array of tiny, light-sensitive diodes and is also now commonly used in video cameras and digital still cameras. Astronomical CCDs, however, are oft tweaked up to provide the all-time functioning at faint light levels, in many cases recording the arrival of individual photons of light from afar sources in the Universe!
Resolving Power versus Spectral Coverage
Every fourth dimension an astronomer goes to a telescope to obtain spectra, he/she has to answer several questions about the goals needed for their investigation. For case, one has to know exactly what spectral lines need to be observed, and hence, how much spectral coverage is necessary. Are all of the lines of involvement in the red role of the spectrum, or is full spectral coverage from the blueish through the reddish needed? The other basic question is how much resolving power is needed (basically, how much does the calorie-free need to be spread out to prove the details in the spectrum)?This terminal question involves several considerations. Are there spectral lines of interest that are close together in wavelength? If so, i must use sufficiently high dispersion to allow the lines to be separated; otherwise the lines will be blended together such that they cannot be measured individually.
Another consideration may be whether one is making velocity measurements. If so, what precision is needed for measuring the redshifts or blueshifts of the lines in the spectrum? For instance, let'south say you wanted to measure velocities of expansion of a planetary nebula, which are typically about 10 kilometers per second, using lines in the ruddy office of the optical spectrum (nigh 6500 Angstroms). The equation for Doppler shifts says you would desire to brand sure your spectrograph can make measurements to an accuracy of at least 0.2 Angstroms.
A difficulty sometimes arises when a project desires both high spectral dispersion and broad wavelength coverage. For a detector of fixed size, the more one spreads the light out (college dispersion) the less the range of wavelengths that will autumn on the detector (smaller spectral coverage). In cases where both spectral coverage and loftier spectral dispersion are needed, a special spectrograph chosen an echelle spectrograph tin can exist used. This device contains two diffration gratings instead of one, a high dispersion grating to provide the desired spectral resolution, and a lower dispersion grating that spreads the overall spectrum out into an array of miniature spectra, each roofing only a portion of the desired spectral range. While these spectrographs are not suitable for every observation, they brand it possible in certain instances for a unmarried observation to do the job of l or more observations with a regular spectrograph!
Click here to go to next section.
Render to Spectroscopy Abode Page.
Bill Blair (wpb@pha.jhu.edu)Source: https://blair.pha.jhu.edu/spectroscopy/measure.html
0 Response to "How to Reading Wavelength Graphs on a Spectroscope"
Post a Comment